INDEX
Atoms will assemble and be a molecule.
Between the molecules and molecular force called “intermolecular force” is working, and then solidified (crystallization).
The chemical bond atom is a like to win the closed-shell structure in search of stable reaction, from this reason, the atoms are you there to make such molecules and crystals.
The resin are mainly the following 4 binding.
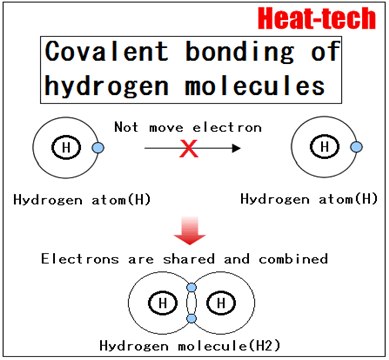
1.Covalent bond
Covalent bond is a chemical bond caused by sharing each other’s electrons between atoms. Bond is very strong, molecules are formed by covalent bonds (single atoms and molecules are excluded). Also, it is a covalent bond crystals crystal, which is formed by a covalent bond. Coordination bond is also a kind of covalent bond.
2.Ionic bond
Ionic bond is a chemical bond by an electrostatic attraction between the anion having a cation and a negative charge having a positive charge.
Ion crystal is formed by this binding.
It is contrasted with covalent bonding, bonding orbital it can also be interpreted as a limit that was localized to the atom of higher electronegativity.
3.Hydrogen bond
A hydrogen bond is the electrostatic attraction between polar molecules that occurs when a hydrogen (H) atom bound to a highly electronegative atom such as nitrogen (N), oxygen (O) or fluorine (F) experiences attraction to some other nearby highly electronegative atom.
These hydrogen-bond attractions can occur between molecules (intermolecular) or within different parts of a single molecule (intramolecular). The hydrogen bond (5 to 30 kJ/mole) is stronger than a van der Waals interaction, but weaker than covalent or ionic bonds. This type of bond can occur in inorganic molecules such as water and in organic molecules like DNA and proteins.
The hydrogen bonds and negative atomic hydrogen atom linked covalently to the vicinity of the lone pair is (nitrogen, oxygen, sulfur, fluorine, π-electron system, etc.) non-covalent attractive forces interaction making the.
Intermolecular hydrogen bonding is responsible for the high boiling point of water (100 °C) compared to the other group 16 hydrides that have no hydrogen bonds. Intramolecular hydrogen bonding is partly responsible for the secondary and tertiary structures of proteins and nucleic acids. It also plays an important role in the structure of polymers, both synthetic and natural.
The substance which does hydrogen band well-melts in water.
That is because by the water and the hydrogen bonding attraction between the solvent and solute become stronger.
4.Van der Waals bond
Molecules are electrically neutral, because it does not have the charge as a whole, never strong Coulomb force, such as work in between the positive and negative ions works between the molecules.
However, if we look molecules microscopically, since a collection of electrons moving around the periphery and the nucleus, there can momentarily electrons in a molecule are distributed unevenly, and therefore in the molecule δ + and δ- of partial charge occurs.
This temporary polarized molecules is allowed to temporarily polarize the molecules adjacent, molecules each other it will be weakly attracted to each other.
This is referred to such interactions between molecules Van der Waals force.
This of Van der Waals forces ties it called the Van der Waals bond.
There is only strength of 1/10-1/1000 compared with something to choose as an ionic bond for the temporary interaction of electrostatics by the intermolecular force.
However, since the number of non-ionic atom of the polymer is enormous, it will have a considerable influence as a binding force and are stacked even chemical bond based on the intermolecular forces.
That is, except in the molecule are tethered molecular bonds, intermolecular forces are expressed to provide the object shape and characteristics of the forces that bind molecules constituting the material.
5.Covalent binding energy
| I – I | 151 kJ/mol |
| F – F | 159 kJ/mol |
| Br – Br | 192 kJ/mol |
| Cl – Cl | 247 kJ/mol |
| H – H | 435kJ/mol |
| O = O | 498 kJ/mol |
| HO – H | 499 kJ/mol |
| CH3 – H | 439 kJ/mol |
| CH3 – CH3 | 385 kJ/mol |
| CH2 = CH2 | 682 kJ/mol |
| N ≡ N | 945 kJ/mol |
Meaning of the symbol -: single bond =: double bond ≡: triple bond
6. The strength of intermolecular forces
| Intramolecular bond | Intermolecular bond | Bond strength ratio | |
|---|---|---|---|
| Covalent bond | ○ | × | Mightiness |
| Ionic bond | ○ | ○ | 1000 |
| Hydrogen bond | ○ | ○ | 100 |
| Van der Waals bond | × | ○ | 1 |
*Van der Waals bond is the value of the single molecule. It will be very powerful in the resin of polymer that the number of molecules is several 100,000 units.
Polymerization shrinkage of the monomer
| Resin name | Polymerization shrinkage ratio |
|---|---|
| Ethylene | 66.0 |
| Vinyl chloride | 34.4 |
| Acrylonitrile | 31.0 |
| Ethylene oxide | 23.0 |
| Methyl methacrylate | 21.2 |
| Vinyl acetate | 20.9 |
| Diallyl fumarate | 17.2 |
| n- propyl methacrylate | 15.0 |
| Styrene | 14.5 |
| Diethylene glycol bis alkali carbonate | 14.0 |
| Epichlorohydrin | 12.0 |
| Diallyl phthalate | 11.8 |
| N- vinylcarbazole | 7.5 |