INDEX
所謂液晶
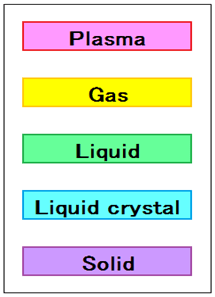
液晶是液體和固體(晶體)之間的中間狀態,或處於這種狀態的物質。
目前,除了表現出液晶結構的樹脂之外,沒有其他產品。
液晶領域正在開發中,新的發現和發明仍在繼續。
固態和液態之間有液晶和柔性晶體。
雖然在三維位置沒有規律性,但液晶在顆粒的取向上具有規則性。
儘管塑料晶體在三維位置具有規則性,但它們在顆粒取向上沒有規律性。
代表性的塑料晶體化合物包括四氯化碳,環己烷和富勒烯。
| 規則性 | 固体 | 液晶 | 柔軟性結晶 | 液體 |
|---|---|---|---|---|
| 三維位置的規律性 | ○ | × | ○ | × |
| 粒子方向的規律性 | ○ | ○ | × | × |
液晶聚合物
表現出液晶結構的樹脂稱為液晶聚合物(LCP)。
液晶基於它們的表達形式分為熱致液晶。
熱致液晶
熱致液晶在熔點和清亮點之間變成“液晶”。
它具有類似液晶的性質,其中分子的線性鏈在熔融狀態下規則排列,並且被定義為熱塑性樹脂。
目前工業上使用的大多數液晶樹脂都是這種類型。
濃度轉移型(溶致)液晶
溶致液晶在溶解於任何液體中之前變成完全溶液之前,在特定濃度範圍內變為“液晶”。
通過溶解的刺激變成液晶,並且當一些表面活性劑以高濃度溶解在水中時可以看到。
在整個溶液中,親水和疏水部分一起形成大規模結構。
在液晶中的四個系統的基礎上有許多階段。
(1). 零維重心順序系統
向列相
手性向列相
盤狀向列相
膽固醇期
(2). 一維周期結構系統
近晶相(縮寫為Sm相)
有許多像SmA,SmB,SmC,並且新發現仍在繼續。
香蕉型近晶相(縮寫為B相)
有許多如B1,B2和B3,並且新的發現仍在繼續。
(3). 二維周期結構系統
Discotic柱狀相
(4). 三維周期結構系統
立方階段
藍相
*液晶中存在各種狀態,新的發現仍在繼續。
液晶的歴史
溶致液晶的発見
Rudolf Ludwig Karl Virchow(1821年10月13日 – 1902年9月5日)是德國病理學家和白血病發現者
1854年,Rudolf Ludwig Karl Virchow,通過將水與髓鞘(生物體的神經組織)接觸,發現了溶致液晶。
熱致液晶的発見
Friedrich Richard Reinitzer(1857年2月25日 – 1927年2月16日)奧地利植物學家和化學家
1888年,F. Reinitzer意外地註意到在布拉格植物生理研究所的一項研究中,膽固醇和苯甲酸酯化合物膽固醇苯甲酸酯解凍了兩次。
當晶體被加熱時,普通固體在一定溫度(熔點)下變為液體。
然而,當加熱膽固醇苯甲酸酯晶體時,它在145.5℃變成混濁液體,並且當加熱時,它在178.5℃變成透明液體。
也就是說,他發現膽固醇苯甲酸酯有兩個熔點。
想知道這件事的F. Reinzer要求O. Rehmann進行詳細調查。
Otto Lehmann萊曼(1922年1月13日,1855年6月13日)德國物理學家
萊曼開發了一種加熱偏振顯微鏡,這是當時最先進的儀器。
Lehmann用加熱的偏振顯微鏡觀察了這種神秘的液體膽固醇苯甲酸酯並證實了這種現象。
我們發現液態膽固醇苯甲酸酯具有雙折射性質,它可以在兩個方向上折射固體晶體的光。
從那時起雙折射已知具有各向異性,這種現象僅在晶體中發生。
雖然它處於液態,但它被認為具有類似水晶的特性,他寫了一篇名為“流動的水晶”的論文。
從這開始,分析了各種材料。
1911年 法國的Charles-Victor-Morgan通過摩擦發現了液晶的排列。
1922年 法國的(George Frieder)建立了三種液晶,即“近晶向列膽甾體”,並被稱為“液晶”,因為它是液體和晶體=液晶之間的中間體。
1963年 美國的RCAR. Williams發現,當向液晶施加電壓時,透明液晶變為不透明。
1968年 美國的RCA向向列型液晶(帶有弦狀圖案的液晶)施加直流或低頻電壓時,會發現液晶透明變為乳白色的現象,被稱為“動態散射效應”並應用於液晶應用。 我順便說一句。
1973年 日本的SHARP將動態散射液晶(DSM)作為顯示器投入實際應用,開發出世界上第一台帶液晶顯示器的計算器,液晶顯示器(LCD,液晶顯示器)將用於各種設備 它成了。
1974年 美國的伊士曼柯達公司開發並推出了“Zyder”,旨在提高聚對苯二甲酸乙二醇酯的耐熱性。
1979年 日本的住友化學株式會社(現為:住友化學)開發了“Econol(現為Sumika Super)”,並根據表面貼裝技術(SMT)提高了耐熱性。
1984年 美國的塞拉尼斯開發了“Vectra”並擴展了其機械部件的範圍。
用於液晶顯示器(Liquid crystal display、LCD)的液晶材料
由液晶的光快門
在液晶中,分子通過電壓和磁力等容易移動,並且光的通過方式改變。
該屬性可用於創建可電控的光閘。
那是Twisted Nematic液晶。
具有扭曲特性的液晶和偏振濾光器的組合,其通常允許光通過,但是當施加電壓時,扭曲消失並且光不通過,因此用作光閘。
這種方法開發的顯示器稱為TN方法,其改進的STN液晶常用於筆記本電腦中一段時間。
在電場上應答的液晶
“向列型”一詞來自“nemato-”,意思是“絲狀”和“線蟲”。
龜殼連續的骨架(通常在絲狀液晶分子中發現)具有其中包含的電子可以相對自由地移動的特性,並且更容易移動,特別是在分子的縱向方向上。
因此,當施加外部電場時,該電子被偏置到靠近正極的一側,導致在分子的縱向上的正或負極化。
接下來,分子的正側被吸引到電場的負值,並且分子的負側被電場的正面吸引,並且分子旋轉到電場。
這是由於電場引起的液晶排列。
液晶結合液體流動性和固體結晶度。
液晶分子可以像液體分子一樣自由移動,但在某個方向上,它們具有像固體晶體原子那樣規則排列的特性。
LCD中使用的液晶是細長的。 例如,稱為5CB的向列型液晶分子具有由連接苯環的硬質部分和其中碳線性連接的軟質部分組成的結構,如下圖所示。
正型液晶
誘電率:長軸方向較大,垂直於長軸方向較小
正型液晶用於TN和IPS類型。
由於柔軟的部分,液晶分子像液體一樣是流體,並且由於硬質部分可以有規律地排列。
當向液晶施加電壓時,氰基(-CN)將液晶分子極化為偏向正電荷的部分和偏向負電荷的部分。
因此,液晶分子規則地排列在電場方向上。 不施加電壓時,不會定期安排。
負型液晶
誘電率:長軸方向小,垂直於長軸方向大
負型液晶用於VA型。
4甲氧基亞芐基-4丁基苯胺N-(4-甲氧基亞芐基) – (4-丁基苯胺)(MBBA)
| 材料名 | 熔點(℃) | 清除點(℃) |
|---|---|---|
| MBBA | 22 | 47 |
| 5CB | 23 | 35 |
| 8CB | 22 | 34 |
結構性質
在向列型液晶中,分子的位置是無限的,它可以自由移動,因此分子容易抵抗外力移動和變形,一旦變形,它就不能自行恢復。
它像普通液體一樣流動,略微粘稠,抗變形。
如果你試圖部分改變分子的方向,返回的力將起作用並抵抗。
這是普通液體中未發現的現象,其電阻根據變形方向而變化,例如縱向彎曲,橫向彎曲或扭曲。
通過將這些特性用於注塑產品,可以製造堅韌的超級工程塑料。
由於在模塑後產生的緊湊晶體結構,它在未增強狀態下比填料增強的工程塑料更硬。
由於在熔融過程中表現出液晶性質,聚合物之間沒有纏結,導致低粘度和模塑期間的優異流動性。
另外,由於成型收縮率和線性膨脹係數低,因此可以處理薄壁結構和精細成型。
這種液晶稱為液晶聚合物(液晶聚合物或液晶塑料(LCP))。
嚴格地說,由於基本結構是對羥基苯甲酸,並且在均聚物的情況下熔點超過600℃的熱分解溫度,芳族聚酯樹脂和液晶聚酯以線性方式與各種組分酯鍵連接。 有人說。
通常,它通過熔融聚合方法製造。
芳族羥基酸的羥基用乙酸酐等乙酰化並加熱以引起脫乙酸縮聚反應以形成線性結構。
還有一種通過熔融聚合製備相對低分子量聚合物並通過固相聚合進一步聚合的方法。
為了尋求耐熱性和流動性之間的相容性,已經研究了LCP用於改進其組成分子,並且已經有超過20種類型的LCP被進一步開發。
“I型”4,4-二羥基聯苯酚和對苯二甲酸與對羥基苯甲酸的縮聚物
Econor住友化學
Zyder Amoko
“II型”2,6-羥基萘甲酸與對羥基苯甲酸的縮聚物
Vectra- Celanese
“III型”對苯二甲酸乙二醇酯與對羥基苯甲酸的縮聚物
Rod Run ・Unitika
Novaculate・三菱化成
LCP的物理特性(典型值)
| 単位 | Ⅰ型 | Ⅱ型 | Ⅲ型 | |
|---|---|---|---|---|
| 比重 | – | 1.60-1.70 | 1.62 | 1.62 |
| 抗拉強度 | MPa | 108 | 206 | 118 |
| 斷裂伸長率 | % | 1.3 | 3 | 5 |
| 拉伸模量 | MPa | 14014 | 9806 | – |
| 彎曲強度(23°C) | MPa | 137 | 152 | 137 |
| 彎曲模量(23°C) | MPa | 12152 | 8820 | 9310 |
| 衝擊強度(Izod / 6.4t缺口) | J/m | 163 | 431 | 392 |
| 硬度(羅克韋爾) | – | – | R66 | R70 |
| 負載變形溫度(1.81MPa) | ℃ | 266-320 | 230-240 | 180-210 |
| 線性膨脹係數(MD方向) | x10-5/℃ | 0.0093 | – | 0.1 |
| 介電常數 | 103/106Hz | 3.56/3.10 | 3.6/3.4 | – |
| 介電切線 | 103/106Hz | 0.0068/0.041 | 0.024/0.018 | – |
| 體積電阻率 | Ω・cm | – | 6×1016 | – |
| 電弧電阻 | sec | 186 | 137 | – |
| 易燃 | mm | V-0, 1.6 | V-0, 0.8 | – |
| 氧指數 | % | 42 | 35 | – |
| 成型溫度 | ℃ | 350-400 | 300-330 | 275-290 |