INDEX
The heat which appears in the temperature of a substance and can be observed is sensible heat.
The heat unobservable although the state of a substance is changed is latent heat.
Sensible heat + Latent heat = Total heat
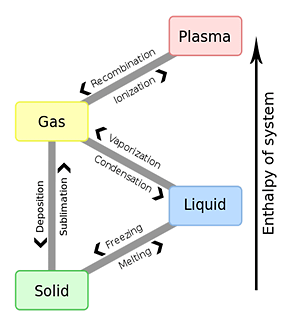
The phase transition of three states of matter.
| From | To | Designation of the phenomenon | Designation of the transition point | Designation of the heat of transition |
|---|---|---|---|---|
| Gas | Liquid | Condensation (liquefaction) | (None) | Heat of condensation |
| Solid | Deposition (Coagulation Solidification, Condensation) | (None) | (None) | |
| Liquid | Solid | Coagulation (Solidification Freezing) | Coagulating point | Heat of solidification |
| Gas | Evaporation (vaporization) | Boiling point | Heat of vaporization (Heat of Evaporation) | |
| Solid | Liquid | Dissolution(Melting) | Melting point | Heat of fusion |
| Gas | Sublimation (evaporation) | Sublimation point | Heat of sublimation |
* Evaporation heat expresses the heat absorbed into a substance, and since positive and heat of condensation are heat which a substance emits, they take a negative value.
2-9-1.Latent heat
Latent heat is a total amount of the thermal energy needed when a substance carries out a phase transition to a liquid or gas from a liquid from a solid.
There are the heat of fusion accompanying dissolution and evaporation heat (vaporization heat) accompanying evaporation in latent heat.
Joseph Black introduced the concept of latent heat.
Black showed that heat was absorbed without changing temperature in 1761, when ice is dissolution, and thought that heat matter (caloric) was connected with icy particles.
Moreover, producing the concept of calorific capacity or specific heat etc.contributed to development of thermology from the fact that the water of the volume and the rise in heat of mercury have a difference etc.
Joseph Black FRSE FRCPE FPSG (16 April 1728 – 6 December 1799) Scottish physician and chemist
2-9-1-1.Latent heat
The heat of fusion is the heat required when the material a certain amount of a phase transition from a solid to a liquid.
The unit is J/g or J/mol, heat of fusion of ice is 333.5J / g.
2-9-1-2.Evaporation heat ( or Vaporization heat )
Evaporation is a process in which the atom or molecule of a liquid state obtains sufficient energy, and will be in a gaseous state.
The evaporation from a liquid takes place at the temperature below the boiling point, and is steam pressure. It continues until it reaches maximum vapor tension, and it reaches and ends to a liquid phase balance there.
If the temperature of a liquid reaches the boiling point, evaporation (boil) will occur also from the inside of a liquid.
Here, a liquid needs evaporation heat (vaporization heat) from the circumference when evaporating.
The molecule with the thermal agitation energy which overcomes the surface tension (intermolecular force) of a liquid can evaporate.
In other words, the evaporating molecule has the dynamic energy exceeding the work function about adhesion on the liquid surface.
Therefore, evaporation advances early more, so that surface tension is so low that the temperature of a liquid is high.
Involved in the evaporation, the entropy of the system is increasing, the flow of energy is required in accordance with the phase change.
To accelerate or to continue the evaporation, a continuous supply of heat energy by the heater is required.
For example, heat of vaporization of helium is low and extremely 0.0845 kJ / mol is, because the van der Waals force acting between helium atoms is very small.
Since the hydrogen bond is working between the water molecules on the other, heat of vaporization is as large as 40.8 kJ / mol, of approximately 5 times the heat capacity when 100 ℃ from 0 ℃ to heating of water (7.53 kJ / mol) it becomes the value.
Although measurement of evaporation heat is made in the boiling point, the value usually rectified by the value of 298K (25℃) is used (since the change by compensation is below an error of measurement, it can be disregarded).
The unit has been used kcal / mol is (kilocalories per mole), but the notation kJ / mol and (kilojoules per mole) is mainstream.
In addition, cautions are required to use evaporation heat for measurement of an intermolecular force.
To work also substance in the gas phase, intermolecular forces will result in a small value is measured than the actual.
2-9-2.Sensible heat
Sensible heat is the quantity of heat spent without changing the state (phase) of a substance in order to change only temperature.
For example, 20℃ water is put into a kettle and it hangs on fire.
Heat was transmitted from fire to a kettle and temperature went up from 20℃ to 60℃.
The heat which water obtained at this time is sensible heat.
Academic definition
In the system which consists of many molecules etc., when the temperature of a system also rises simultaneously with the increase in internal energy, this microscopic dynamics energy is called sensible heat.