INDEX
Check the performance of the air and nitrogen as the medium to be used in the hot-air drying.
4-1-1.The role of air
Air at room temperature, the atmospheric pressure will behave as a nearly ideal gas.
Density of air ρ [kg / m3] at t [℃], atmospheric pressure P [atm], the water vapor pressure e [atm] ,
![]()
The advantages and the disadvantages of the air used for hot air heating.
◎ Low cost
◎ Safe into leaks from pipes
◎ Warm the material as a heat source
◎ Reduce the gas boundary layer membrane
◎ Convey the water vapor (carrier gas)
○ Includes several percent water vapor, the effect of water vapor is a positive effect on the direction of air in the normal environment.
○ Oxygen is combined with the aluminum components of the heating coil, acting on the extension of the life of the coil to form a corrosion-resistant coating on the surface.
× Air includes oxygen, which increase oxidation at high temperatures.
4-1-2.The role of nitrogen
The advantages and the disadvantages of the nitorogen used for hot wind heating.
◎ Warm the material as a heat source
◎ Oxygen-free environment is prevent the fire.
◎ Oxygen-free environment is prevent oxidation.
◎ Non-vapor environment is prevent the hydroxyl group or prevent hydrolysis.
◎ Reduce the gas boundary layer membrane
◎ Convey the water vapor (carrier gas)
○ Nitrogen is a relatively safe even if leakage.
× Heating efficiency will be lower by the difference between the room temperature if it to vaporize the liquid nitrogen.
× It is not forming a corrosion-resistant coating on the surface of the heating coil because there is no oxygen, the life of the coil will be shorter as compared to the air
Since nitrogen is non-oxygen, it suits dryness of the inflammable substance containing a solvent.
4-1-3.Density of air
When the air define gas as 4 nitrogen and 1 oxygen, so nitrogen molecular weight 28 and oxygen molecular weight 32, the molecular weight of the air will be 28.8.
Calculation for determining the density of air ( Pressure 1atm, Room temperature 20 ℃ )
Pressure p = 1atm = 101.3×10 ^ 5Pa
Temperature t = 273 + C
Air V = 1m ^ 3
Number of moles n = mol
Gas constant R = 8.31J / mol · K
Ideal gas equation = pV = nRT
Density of air ρ =
n = pV/RT = 1.103x10^5Pa x 1m^3 / 8.31J/mol・K x (273+20)K = 41.6mol
ρ= (28.8 x 41.6)g/m^3 = 1200g/m^3 = 1.2g/ℓ
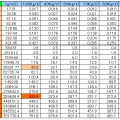
4-1-4.Characteristics List
Constituents of air
| Component | Chemical formula | Volume ratio percentage | Mass ratio | Gaseous density | Specific gravity |
|---|---|---|---|---|---|
| Nitrogen | N2 | 78.084 | 0.75520000 | 1.251 | 1.105 |
| Oxygen | O2 | 20.9476 | 0.23150000 | 1.429 | 0.967 |
| Argon | Ar | 0.934 | 0.01280000 | 1.78 | 1.38 |
| Carbon dioxide | CO2 | 0.039 | 0.00046000 | 1.977 | 1.54 |
| Neon | Ne | 0.001818 | 0.00001200 | 0.9002 | 0.69 |
| Helium | He | 0.000524 | 0.00000072 | 0.1785 | 0.13 |
| Methane | CH4 | 0.000181 | – | – | – |
| Krypton | Kr | 0.000114 | – | – | – |
| Sulfur dioxide | SO2 | 0.0001 | – | – | – |
| Hydrogen | H2 | 0.00005 | – | – | – |
| Dinitrogen monoxide | N2O | 0.000032 | – | – | – |
| Xenon | Xe | 0.0000087 | – | – | – |
| Ozone | O3 | 0.000007 | – | – | – |
| Nitrogen dioxide | NO2 | 0.000002 | – | – | – |
| Iodine | I2 | 0.000001 | – | – | – |
Outline air composition
| Component | Chemical formula | Volume ratio percentage | Mass ratio |
|---|---|---|---|
| Nitrogen | N2 | 0.79 | 0.768 |
| Oxygen | O2 | 0.21 | 0.232 |
Characteristics of the constituents of the air
| Component | Van der Waals constant | Molecular weight | Gas constant | Specific heat at constant pressure | Specific heat at constant volume | Specific heat ratio | |
|---|---|---|---|---|---|---|---|
| a / Pa m6 mol−2 | b / m3 mol−1 | g/mol | R (J/g・K) | Cp (J/g・K) | Cv (J/g・K) | γ | |
| Air | 135 × 10−3 | 36.6 × 10−6 | 28.967 | 0.28703 | 1.005 | 0.718 | 1.399 |
| Nitrogen(N2) | 141 × 10−3 | 39.2 × 10−6 | 28.013 | 0.29680 | 1.039 | 0.743 | 1.399 |
| Oxygen(O2) | 138 × 10−3 | 31.9 × 10−6 | 31.999 | 0.25984 | 0.914 | 0.654 | 1.398 |
| Water vapor (H2O) | 553 × 10−3 | 33.0 × 10−6 | 18.015 | 0.46152 | 4.186 | 3.147 | 1.33 |
| Helium(He) | 3.45 × 10−3 | 23.8 × 10−6 | 4.003 | 2.07727 | 5.240 | 3.16 | 1.66 |
| Argon (Ar) | 136 × 10−3 | 32.2 × 10−6 | 39.948 | 0.20813 | 0.523 | 0.32 | 1.68 |
| Hydrogen(H2) | 24.8 × 10−3 | 26.7 × 10−6 | 2.016 | 4.12449 | 14.250 | 10.12 | 1.408 |
| Carbon monoxide(CO) | 151 × 10−3 | 40.0 × 10−6 | 28.010 | 0.29684 | 1.041 | 0.74 | 1.4 |
| Carbon dioxide(CO2) | 365 × 10−3 | 42.8 × 10−6 | 44.010 | 0.18892 | 0.819 | 0.63 | 1.3 |
| Ammonia(NH3) | 422 × 10−3 | 37.1 × 10−6 | 17.031 | 0.48821 | 2.060 | 1.57 | 1.31 |
| Methane(CH4) | 238 × 10−3 | 42.8 × 10−6 | 16.042 | 0.51828 | 2.160 | 1.63 | 1.32 |
The specific heat is the amount of heat per unit mass required to raise the temperature by one degree Kelvin.
The characteristics of the air
| Temperature conditions | Density | Specific heat at constant pressure | Coefficient of viscosity | Kinematic viscosity | Thermal conductivity | Thermal diffusivity | Prandtl number |
|---|---|---|---|---|---|---|---|
| T | ρ | Cp | η | ν | λ | α | Pr |
| K | kg/m3 | kJ/(kg・K) | μPa・s | mm2/s | mW/(m・K) | mm2/s | – |
| 100 | 3.6109 | 1.072 | 7.1 | 1.97 | 9.22 | 2.38 | 0.826 |
| 200 | 1.7679 | 1.009 | 13.4 | 7.58 | 18.1 | 10.15 | 0.747 |
| 300 | 1.1763 | 1.007 | 18.62 | 15.83 | 26.14 | 22.07 | 0.717 |
| 400 | 0.8818 | 1.015 | 23.27 | 26.39 | 33.05 | 36.93 | 0.715 |
| 500 | 0.7053 | 1.031 | 27.21 | 28.58 | 39.51 | 54.33 | 0.71 |
| 600 | 0.5878 | 1.052 | 30.78 | 52.36 | 45.6 | 73.7 | 0.71 |
| 700 | 0.5038 | 1.076 | 34.1 | 67.7 | 51.3 | 94.6 | 0.715 |
| 800 | 0.4408 | 1.099 | 37.23 | 84.5 | 56.9 | 117 | 0.719 |
| 900 | 0.3918 | 1.122 | 40.22 | 102.7 | 62.5 | 142 | 0.722 |
| 1000 | 0.3527 | 1.142 | 43.08 | 122.1 | 67.2 | 167 | 0.732 |
| 1100 | 0.3206 | 1.16 | 45.84 | 143 | 71.7 | 193 | 0.742 |
 HEAT-TECH Best Technology Online Shop
HEAT-TECH Best Technology Online Shop