There is a limit to the amount of water vapor that air can contain.
Within the air, water movement may become gas (vapor) from a liquid, that or become liquid from gas (vapor) has been constantly repeated.
The temperature that is the amount of energy possessed by the air.
Air temperature is high, that is in the air a large amount of energy, water molecules and air molecules are moving violently.
The movement of these molecules are in the air temperature is low in the reverse is weak reduced.
The temperature is high, that energy is large, the air in the activity of the molecule is active, rather than liquid, the number which is present as water vapor in the gas is the increased water molecules.
The temperature is low in reverse, energy is small and in the air activity of the molecule is weak, the amount of water molecules are present as water vapor will be less.
Difference in this amount, it appears as the difference in the amount of water vapor contained by the temperature.
And it called the saturation state containing vapor to the limit.
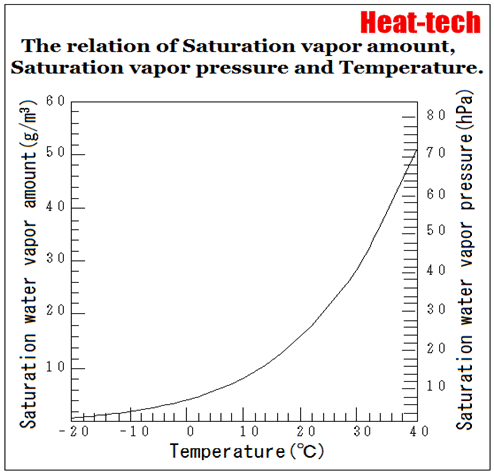
Saturation amount of water vapor will be more at higher temperatures.
Temperature increases, saturation vapor amount (saturated water vapor pressure) will increase quadratically.
It is called the saturation water vapor amount of water vapor content at that time, it represents what g water vapor on whether contained in the air of 1m3.
It may also be represented (partial pressure, hPa) at a pressure = saturated water vapor pressure of water vapor in place of saturated water vapor.