INDEX
What is a radiation thermometer?
The palm feels warm when user point it at the sun, because the palm’s warm nerve cells sense the infrared radiation emitted by the sun.
The temperature information sensed by the warm nerve cells is transmitted to the brain through the nerves and recognized as “hot”.
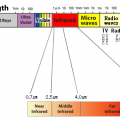
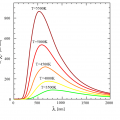
Not only the sun, but all substances emit infrared light in proportion to the temperature of the substance.
A radiation thermometer measures the temperature by capturing the infrared radiation emitted from a substance.
Principle of radiation thermometer
The radiation thermometer uses a thermopile.
Thermopile is a detection element that absorbs infrared rays radiated from a substance and generates an electric signal according to temperature.

Thermopile-SEMITEC Corporation
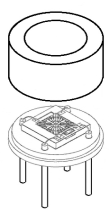
The thermopile is equipped with a large number of thermocouples, collecting hot junctions in the center and cold junctions in the periphery.
The infrared rays emitted from the substance are focused on the thermopile by the lens and the hot junction is heated.
The Seebeck effect creates a voltage difference between the hot and cold junctions, allowing temperature measurement.
This is amplified by an amplifier, the emissivity is corrected, and the temperature is displayed.
Features of radiation thermometer
The main feature of the radiation thermometer is that it measures temperature without contact.
Key advantages of using a radiation thermometer
Temperature measurement of moving / rotating substances
Temperature measurement of a substance that causes a crack on the surface when a thermocouple is contacted
Temperature measurement of small heat capacity materials whose surface temperature changes when a thermocouple is brought into contact
Key disadvantages of using a radiation thermometer
Emissivity setting is essential according to the substance
Unable to measure temperature inside object
Unable to measure gas temperature
Points when using radiation thermometer
About emissivity
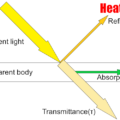
Since the emissivity ε of heat radiation differs depending on the heating object, the measurement temperature cannot be measured correctly unless the correct emissivity is set.
Emissivity defines a blackbody as 1.
For rubber and ceramics, 0.95 will be enough.
Heated objects with a glossy surface, such as metals, generally have low emissivity.
How to determine emissivity
If the emissivity of the object to be heated is roughly acceptable for the method of using for reproducibility, refer to the emissivity described in the literature.
According to Kirchhoff’s (radiant energy) law, the energy absorbed by a substance is equal to the energy emitted, so the absorption rate = emissivity.
Reference site Far infrared absorption rate
If an accurate emissivity is required, measure the temperature using two methods, a radiation thermometer and a contact thermometer such as a thermocouple, and set the emissivity so that the displayed values match.
Emissivity setting error
If the emissivity set by the radiation thermometer is different from the correct emissivity of the measurement object, it may cause measurement errors.
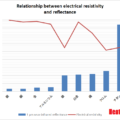
The relationship between the emissivity and the temperature of the measurement object is not a straight line, so the measurement temperature will change if the measurement conditions differ.
A 1% difference in emissivity setting does not mean a 1% difference in temperature.
Sometimes it’s more, sometimes less.
Relationship between emissivity setting error and temperature measurement error (typical example)

Measurement object size, spot diameter and measurement distance
Radiation thermometers have a measurable range (called spot diameter) and measurement distance.
For accurate temperature measurement, the spot diameter and measurement distance must be used at the rated value.
When using a radiation thermometer, the spot diameter must always be smaller than the object.
 HEAT-TECH Best Technology Online Shop
HEAT-TECH Best Technology Online Shop