Every substance is made up of very small particles. The molecules that make up that substance have different molecular motions depending on the temperature. A molecule is made up of several atoms linked by chemical bonds, and any bond. Each also has its own frequency. Light that normally resonates with the frequency of the vibration of the bond increases the molecular motion and raises the temperature of the substance, which is similar to the shaking of a building in an earthquake. When the period of and the natural period of the building match, the shaking of the building becomes stronger.
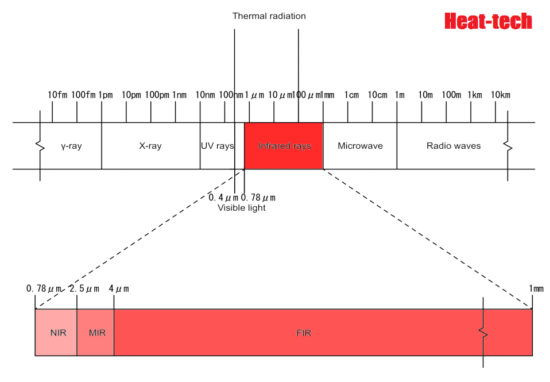
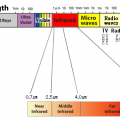
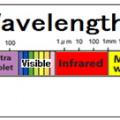
Infrared light has a longer wavelength than visible light, from 0.78μm to 1mm. When infrared rays of light emitted from a light source enter a substance, the substance reflects”, “absorbs”, and “transmits” the light rays. If the absorbed light beam matches the frequency of the molecular bond, the vibration will be increased. Molecules constantly vibrate, and since this vibration is the thermal energy itself, the thermal energy increases as the vibration increases. ”
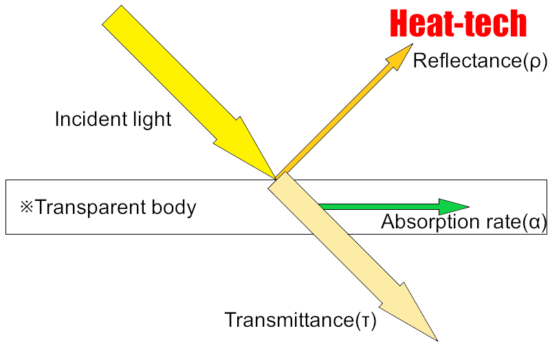
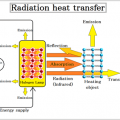
The sum of the reflectance, transmittance and absorption of energy incident on a certain plane is always 1.
Reflectance (ρ) + Transmittance (τ) + Absorption rate (α) = 1 (incident light)
This is because, according to the law of conservation of energy, the total amount of energy before conversion and energy after conversion is always invariant.
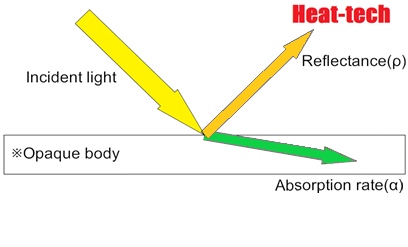
Since most objects are opaque objects, the transmittance (τ) = 0, and only the reflectance and absorption.
Reflectance (ρ) + Absorption rate (α) = 1 (incident light)
According to Kirchhoff’s law, at the time of thermodynamic equilibrium, absorption rate ελ = emissivity αλ.
Therefore, a substance with a high absorptivity also has a high emissivity.
All objects emit electromagnetic waves of all wavelengths depending on their absolute temperature. When this electromagnetic wave is absorbed by another object, it is converted into heat energy, and the temperature of the object rises. Of these electromagnetic waves, the wavelength of thermal radiation is in the range of approximately λ0.4 μm (visible) to λ100 μm (far infrared rays). The heat radiation of an object changes depending on the plane temperature and plane condition. Rough planes with irregularities have better absorption than polished planes.
 HEAT-TECH Best Technology Online Shop
HEAT-TECH Best Technology Online Shop